Module 2
How to Participate
To participate in this training module, complete the following activities in the prescribed order:
- Complete the Pre-Test
- Read the training materials
- Complete the Post-Test

Overview
Module 2 consists of 3 sections:
Part A: Programmatic implications of COVID-19 vaccines
Part B: Managing storage, transport, and delivery of COVID-19 vaccines
Part C: Cold-chain management at the vaccination post and waste management at the facility level
Part A: Programmatic implications of COVID-19 vaccines
Learning Objective
At the end of this section, you should be:
- able to identify key programmatic considerations that are new or different for COVID-19 vaccines; and
- able to describe with key planning elements to prepare for COVID-19 vaccine.
What is different about COVID-19 Vaccination Logistics?
Stability Data of Candidate Vaccines Is Not Yet Available
Currently, the information on the stability of candidate vaccines is either limited or not yet available. We don't know if the vaccines will have Vaccine Vial Monitor or VVM and if they do, what type. We don't know which vaccines will qualify for the multi-dose vial policy or MDVP. We also don't know the vaccine shelf life. We don't know which vaccine will be sensitive to light and freezing. It is likely that some vaccines will only have information on manufacturing date instead of expiration date. The information on vaccine expiration is likely to be available in the product information leaflet. Therefore, health workers are encouraged to refer to the package insert which may be available through a QR code or manufacturer's website link.
WHO prequalification status of vaccines No COVID-19 vaccines are currently pre-qualified by the World Health Organization, but it is possible that some vaccines may be pre-qualified during the deployment period. Therefore, most vaccines will be used under the WHO Emergency Use Listing or EUL procedures.
What is EUL? It is a process developed by WHO to expedite the availability and use of unlicensed medical products needed in public health emergency situations. Click here to learn more about EUL.
Vaccines may or may not have VVM on the label or cap The location of the VVM on the vial, that is either on the label or cap, will depend on the vaccine stability profile and suitability to multi-dose vial policy. If VVM is available, health workers are encouraged to follow the VVM guidance below to decide which vaccine to use or discard:
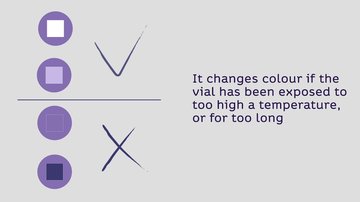
Planning for a Successful Introduction
Having a carefully designed supply chain and logistic plan is key to ensure that COVID-19 vaccines will be available in the right quantity and quality, at the right place and at the right time. Health workers are encouraged to use the "How to prepare for COVID-19 vaccine management" checklist as guidance.
Preparation for vaccine arrival
- Ensure efficient internal communication and information sharing. This is important because new information may be available that are critical for health workers to know to effectively manage COVID-19 vaccine during the deployment and vaccination period.
- Maximize use of a traceability system, such as barcode and/or QR code. The primary purpose of the barcode is to track vaccine movement to reduce the risk of fraud or diversion. While the QR code will be used as one of the means to access information about the vaccine and update health workers on new information and guidance that may help improve their management of the vaccine and maximize the vaccine potency. Health workers are therefore encouraged to keep the vaccine in their secondary packaging to enable scanning of the barcode and QR code.
Transportation
- Ensure appropriate equipment and coolant-packs.
- Using temperature dataloggers in each transport box is preferred.
Storage
- Install reliable continuous temperature monitoring system.
- Monitor temperature more frequently (more than 2 times a day).
Timing
- Maximize COVID-19 vaccinations to reduce wastage.
Part B: Managing storage, transport, and delivery of COVID-19 vaccines
Learning Objectives
At the end of this section, you should be able to:
- explain cold-chain requirements for COVID-19 vaccines and diluents; and
- explain storage principles/conditions at the health facility as well as for outreach and campaign vaccination posts (e.g. for vaccines and diluents).
Storage Requirements for COVID-19 Vaccines
COVID-19 vaccines candidates currently being developed can be categorized by three storage requirements (as of December 2020):
Vaccines required to be stored at +2 to +8 ºC: The WHO guidance for managing vaccines will apply.
Vaccines required to be stored at -20 ºC: Requires significant investment in ultra-cold-chain (UCC) storage and transport capacity.
Vaccines required to be stored at -70 ºC: Training on handling of vaccines will be necessary.
Cold chain surge capacity options to manage COVID-19 vaccine
+2 to +8 °C
Standard Procedures
- Map all cold-chain storage points (both public and private) at this temperature range.
- Conduct a gap analysis to determine cold-chain storage needs.
If storage capacity is insufficient, consider:
- procurement of solar direct drive (SDD),ice-lined refrigerators (ILRs) and/or cold boxes and vaccine carriers;
- leasing of a private facility;
- splitting shipments and increasing distribution frequency; and
- staggering campaigns.
-20 °C
Standard Procedures
- Map all cold-chain storage points (both public and private) at this temperature range leveraging all polio vaccine infrastructure.
- Conduct a gap analysis to determine cold-chain storage needs.
If storage capacity is insufficient, consider:
- procurement of freezers, cold boxes, and vaccine carriers;
- leasing of a private facility;
- splitting shipments and increasing distribution frequency; and
- staggering campaigns.
-70 °C
Most health workers are not familiar with managing vaccines at this temperature range. Follow the recommended strategy for managing ultra-cold-chain equipment, including recommended coolant-packs and use of personal protective equipment (cryogenic gloves).
How to manage COVID-19 vaccines stored at +2 to +8 ºC?

- Ensure all vaccines and diluents are kept in their original secondary packaging and are clearly labeled.
- Whenever possible, keep both vaccine and diluent in the same refrigeration unit.
- Monitor and record temperature more frequently (>2x/day).
- Investigate any fluctuation of temperature and correct the root cause as soon as possible.
- Use a labeled container or tray for loose unopened vaccine vials to facilitate inventory.
- Follow the “first manufacture/expiry, first out” principle: use first the vaccine with earlier manufacturing/expiry date and/or darker VVM (if available) to minimize wastage.
- Record and discard all wastage of vaccines immediately according to national guidelines.
- Review and update contingency plan in case of power failure, equipment breakdown, or cold chain breach.

- Do not return opened vaccine vials to the cold chain. Discard them according to existing standard operating procedures (SOPs).
- Never put vaccines in contact with or close to the evaporator plate in the refrigerator. *Since heat/freeze sensitivity information is not fully known, keep vaccine vials in the middle section, especially if the refrigeration unit has a freezer compartment, to avoid freezing the vaccine.
- Do not keep reconstituted vaccines for more than 6 hours after opening or after the end of an immunization session, whichever comes first – this may change once vaccine stability information is available.
How to manage COVID-19 vaccine stored at -70 ºC?

In addition to general cold-chain management principles for +2 to +8ºC, when handling vaccines stored at UCC, it is also essential to:
- follow existing guidance specific for UCC management;
- use appropriate personal protective equipment (e.g. cryogenic gloves) for personnel in charge of managing UCC; and
- follow guidance and apply best practices for managing and disposing of used phase-change material (PCM).
All responsible personnel (supply chain/ cold-chain personnel, vaccination team and supervisors) should be trained and demonstrate ability to manage UCC according to SOPs

- Do not return opened vaccine vials to the cold chain. Discard them according to existing guidance.
- Do not store laboratory specimens, drinks, food products, or expired health products with vaccines.
- Do not keep vaccines with VVM that have reached the discard point.
- Do not keep expired vaccines in the refrigerator.
What types of supplies/equipment are needed for UCC?
UCC equipment is costly and not recommended to be installed in every facility.
- Establishing a UCC hub at national and strategically at sub-national level is a cost-effective approach.
- Utilizing available cold-chain or UCC facilities from private sectors with appropriate training and monitoring system is a practical approach.
Use of long-term passive containers and thermal shippers to store vaccines and to conduct immunization session is favourable at facility level.
UCC supplies/equipment recommended at the facility level:
- cryogenic gloves
- Arktek + PCM
- thermoshippers + dry ice
- dry ice box + dry ice.

There are 2 types of Arktek passive vaccine storage device
- Arktek passive vaccine storage device: a super-insulated container optimized to safely store vaccines between 0 to +10 ºC for 35 days or more, using only coolant-packs in hot zone conditions.
- Arktek + PCM: a modified version of the Arktek device that uses phase-change material rather than coolant-packs to maintain a cold environment for up to 6 days.
Specific PCM packs are frozen around –70 °C and have stable thermal characteristics.

Phase-change materials require special considerations
Phase-change materials (PCMs) are substances that improve thermal performance when applied to a cold-chain product by transitioning from solid to liquid or vice versa.
Optional Resource: Phase-Change Materials for Vaccine Cold Chain Applications Summary Report.

Human Health Risks:
- Health hazards posed by the PCMs are minimal if their primary container is intact.
- Health hazards that can occur if PCMs leak out of their primary container are: potential serious eye irritation from leaking S8 salt hydrate and potentially fatal complications from aspiration/ingestion of paraffin PCM.
Environmental Risks:
- The PCM CrodaTherm™ 5 is found to be toxic to some aquatic life.
- Most other PCMs were biodegradable and none were environmentally persistent, bio-accumulative, or contained toxic chemicals.
- To minimize the risk, release of the PCMs into the environment should be avoided.
Managing transport and delivery of COVID-19 vaccines

General considerations when transporting vaccines
- Plan route of vaccine delivery and time to each destination to include time for checking temperature based on existing guidance.
- Assess potential risk to delivery personnel and delay delivery for foreseeable reasons (e.g. weather conditions), and include risk mitigation strategy in the plan.
- Ensure reliable transport and sufficient funds for fuel. Refrigerated vehicle equipped with datalogger is preferred; consider renting, if needed.
- Bundle supplies when delivering to ensure all logistics are available for vaccination.
- Ensure required documentation is duly prepared and available before departure.
- Never transport multi-dose vials that are opened. Discard open multi-dose vials.

Transporting COVID-19 vaccine at +2 to +8 ºC
- Use only recommended transport boxes with adequate and appropriately prepared coolant-packs.
- Monitor temperature, preferably using dataloggers, as this allows monitoring throughout the transport period and download of data at the end of the trip.
For the datalogger inside the container: check the temperature at the end of the trip (avoid exposing vaccines to temperature change from frequent openings).
For dataloggers with outside reader: check temperature at least 2 times during the trip.
- Complete deliveries in daytime if possible to reduce risk of robbery and/or consider the safety of delivery personnel.

Transporting COVID-19 vaccine at UCC (-70 ºC ) When transporting vaccines in UCC, one of the following specialized containers with dataloggers are recommended:
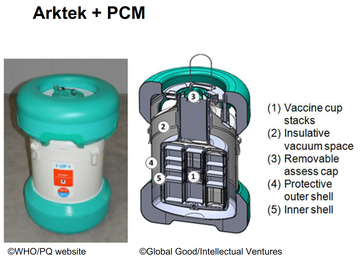
OR

Part C: Cold-chain management at the vaccination post and waste management at the facility level
Learning Objectives
At the end of this section, you should be able to:
- identify and describe actions in case of insufficient cold-chain capacity at the health facility level; and
- describe waste management options and comply with procedures.
Managing cold chain at the vaccination post
Vaccine carriers (VC) For vaccines stored at +2 to +8 ºC, there are 2 different types of vaccine carriers you may use:
- Standard VC without a barrier separating the vaccine storage compartment from the conditioned ice-packs;
- Freeze-preventive VC with a barrier separating the vaccine storage compartment from the frozen ice-packs.

Vaccines stored at -70 ºC require thermal shippers. Thermal shippers are identified by these characteristics:
- Specific vaccine carriers requiring dry ice
- Temperature range: -80 to -60 ºC
- Capacity: 3.4 to 6.2 litres
- With vial rack system
- Built-in temperature datalogger

Handling COVID-19 vaccine vials after opening
- Always check for latest information about the suitability of vaccine to the MDVP.
- As a precaution to maintain vaccine safety and quality, the following are recommended until more information becomes available: For opened vials, if possible, use a separate vaccine carrier with a temperature monitoring device to allow monitoring of temperature during use. Reconstituted vaccine should be discarded 6 hours after opening or at the end of immunization session, whichever comes first.
- Do not use opened vials if temperature in vaccine carrier is found to exceed recommended range – discard vials and replace the coolant-packs as needed.
- Never expose opened vials to direct heat, light or sunlight.
- Never transport or return opened multidose vials used in vaccination to the cold chain – discard them
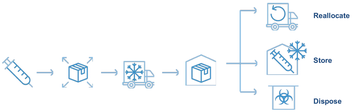
What is reverse logistics? When would you use reverse logistics?
Reverse logistics is the process of retrieving unused vaccines either to dispose or reuse. Possible reasons to conduct reverse logistics:
- Need to re-allocate vaccines to higher-risk areas based on latest epidemiological information
- Return of unused vaccine to higher-level store at the end of vaccination campaign (if relevant)
- During temporary pause of vaccination campaign for any reason
- To recall the vaccine for any reason
Healthcare Waste Management at the Facility Level
Disposal of Syringes
- Use auto-disable (AD) syringes and dispose of them as sharps waste.
- Without recapping the needle, discard the used syringe into the safety box or safe syringe container.
- Do not fill the safety box more than ¾ of its capacity or up to the red line marked on the container.
- Ensure the box is properly labeled with the infectious substances symbol.
- Seal the safety box before transporting it to the treatment site.
Disposal of Vials
- Put used vaccine vials and unopened vaccine vials which have expired or suffered heat exposure into a red or yellow bag for infectious waste, or into a biohazard container.
- Open vials posing a risk of cuts may be classified as sharps waste.
- Ensure that bags/containers are properly labeled with the infectious substances symbol.
- Seal the containers before transporting them to the treatment site.
Disposal of PPE
- PPE includes single-use gloves, aprons and gowns, surgical masks, face protectors in the form of glasses, goggles or face shields.
- Use a room/place away from the vaccination area to remove all used PPE.
- Consult the national guidelines to follow special procedures for removing PPE.
- After safely removing used PPE, put them into a special waste container or bag for infectious waste (yellow or red).
- Ensure that bag/container is properly labeled with the infectious substances symbol.
- Seal the containers before transporting them to the treatment site.
Key considerations for used PCM
- PCM is held in plastic or metal containers and as long as the containers are intact, the PCM should not enter the environment.
- But, plastic and metal will degrade over time and the ambient conditions of many cold-chain equipment (CCE) disposal locations (e.g. open-air landfills or other outdoor areas) will speed the degradation or facilitate puncturing of the container.
- Therefore, PCM should ideally be drained for proper disposal at the end of their useful life.

Additional Resources
- Policy brief on health products traceability
- Prequalification Resource 1
- Prequalification Resource 2
- Waste Management Resource 1
- Waste Management Resource 2
- Waste Management Resource 3
- Ultra-cold chain/Phase-change materials
- Learning from Ebola and Measles Resource 1
- Learning from Ebola and Measles Resource 2
Average Rating: ☆ ☆ ☆ ☆ ☆ (0 reviews)




